About us
With 20 years of experience, our expertise spans the AI lifecycle - from data engineering and AI agent development to cloud integrations and human-centric product design.
Learn more
Qubika at Databricks Data + AI Summit
Join us June 9-12 to see our leading Databricks capabilities
Our Studios
Our Studio delivery model enables us to address challenges head-on by bringing technology and domain experts together. This ensures we deliver immediate business value with our customized solutions.
Learn moreQubika Studios
Product Design
UX research, service design, design thinking, and UI design.
Artificial Intelligence
Agentic AI, GenAI, machine learning, NLP, computer vision.
Data
Data manipulation, engineering, visualization, and prediction.
App Solutions
Native or hybrid, SDK development, integrations, app store positioning.
Cloud, SRE, & DevOps
Cloud migration, CI/CD pipeline development, SRE, infrastructure-as-code.
Cybersecurity
Secure SDLC, AI-powered cybersecurity, vCISO, penetration testing, AI security assessments.
Platform Engineering
Robust infrastructures, scalable APIs, efficient deployment.
Quality Assurance
AI-augmented QA, test automation, CI/CD, load and performance testing, data testing.
Embedded Engineering
Development for semiconductors, embedded systems, IoT, & microcontrollers.
Product Management
Product consulting, process management, monetization.
Blockchain
Smart contracts, decentralized apps, blockchain integration.
Industries
Qubika partners with leading organizations across industries, delivering technology solutions that drive transformation and measurable results. Our expertise empowers clients to achieve business goals through tailored digital strategies.
Our Industries
Banking
Modernize banking systems for a secure, compliant, AI-ready enterprise ecosystem.
Financial Services
Secure, data and AI-driven financial services - from paytech and financial infrastructure to risk, compliance and analytics.
Health & Wellbeing
People-centric healthcare solutions, from virtual care to integrations and smart devices.
Insurance
AI-powered insurance solutions - from accelerating policy lifecycle management to accelerating claims processing.
Media & Entertainment
AI-native solutions to deliver personalized, real-time, and immersive experiences at scale.
Hi-Tech & Semiconductors
Semiconductor design, firmware, and IoT development, AI-powered embedded systems.
Industry Insights
Impact Studies
Explore in-depth case studies showcasing how Qubika empowers organizations to lead, innovate, and transform their industries. Your journey begins here.
Learn moreAvant
Explore how Qubika and Avant are building a new generation of data and AI-driven financial services for their 3 million+ customers.
OnePay
Qubika is a transformational partner to Walmart's fintech, ONE, creating an all-in-one financial experience for its 1 million+ customers.
Shopify
Qubika worked with one of the largest multinational e-commerce companies, Shopify, to transform the digital merchant and retail experience.
MyRow
Explore how Qubika applied its AccelerateAI framework with MyRow to harness AI-driven development and significantly improve speed-to-value.
Tabula Rasa
Tabula Rasa leveraged agile product management to revolutionize drug traceability, streamline processes, and achieve a rapid market launch.
YouScience
The Qubika Data Studio used machine learning to create hyper-personalized career paths for students on the YouScience edtech platform.
Computer Vision
Qubika is a leading provider of computer vision solutions. These case studies show how we're using AI to build innovative products that are transforming lives.
Wearables
Qubika is at the forefront of the wearable revolution. See a selection of our case studies.
Insights
Dive into our expert insights on the latest in technology and business developments.
Learn moreKey Categories
More Insights
Highlighted Posts
Latest Posts
Partners

About us
With 20 years of experience, our expertise spans the AI lifecycle - from data engineering and AI agent development to cloud integrations and human-centric product design.
Learn more
Careers
Are you interested in shaping the AI-future? Then discover how you can join one of the fastest growing companies in the industry.
Learn more
Locations
Qubika's locations are centers of high-quality innovation. Explore our global network and get in touch with our experts.
Learn moreDatabricks Solutions
We've empowered numerous industry leaders to harness the full potential of Databricks' Intelligence platform, driving transformative results and measurable business impact.
Highlights of our partnership
Select Tier Partner
Qubika is a Select Tier Databricks partner, delivering scalable, secure data and AI solutions on Databricks.
200+ certified Databricks engineers
Qubika has 200+ certified engineers on the Databricks Intelligence Platform
20+ years of data experience
Qubika has been providing end-to-end data services for over 20 years.
Databricks Insights

In response to stringent pharmaceutical regulations, Tabula Rasa Healthcare aimed to improve drug traceability using a Chain of Custody (CofC) application for medication delivery monitoring.
They partnered with Qubika to overcome challenges including tight timelines and evolving specifications. With close collaboration between design and development teams, we achieved a successful launch fostering enhanced pharmaceutical traceability.
Studios involved
Deliverables

Given the increasingly stringent regulatory landscape, with measures such as the Drug Supply Chain Security Act, pharmaceutical providers are under increasing pressure to guarantee traceable, uniquely-labeled drug packaging. Such a system, marked by a unique serial number and a corresponding database, allows for the tracking of products throughout the entirety of the distribution supply chain.
In response to this need, Tabula Rasa Healthcare, a healthcare technology provider, sought to broaden its service offering by facilitating end-to-end drug traceability through an intuitive web application, Chain of Custody (CofC). This innovative application empowers the PACE (Program of All-Inclusive Care for the Elderly) programs to trace, record, and audit the various stages of medication delivery. This meticulous documentation process follows the product’s journey from the pharmacy to the PACE centers, then to the transport driver, the front-desk receptionist, and eventually the end user.
However, they encountered several hurdles during this mission.
Firstly, they were working within a tight timeframe, with the pressure to bring the product to market promptly.
Secondly, they were dealing with a product specification that was still in the process of being finalized. This meant they needed assistance in both product design and management to incorporate and balance the bold ideas they wanted to include.
Finally, they had to navigate the rigid requirements of building a product that complied with regulatory standards and a predefined API specification. In other words, they needed to validate critical product decisions with their product owner and key stakeholders before proceeding with the software development.

To navigate these challenges, the team at Qubika employed an Agile Product Management strategy. This approach broke down the high-level specification into manageable ‘epics’ or component functionalities, facilitating a smoother development process. We established iterative feedback loops, allowing for real-time interface with the client during the product’s development, conducted by our development team.
The Agile approach ensured that the product development process was always one iteration ahead of the software development. This was achieved by having the Scrum development team work in parallel with the product design. By doing so, the possibility of wasted development time due to rework was significantly reduced.
This strategy proved instrumental in fostering collaboration between the product owner, the design team, and the development team. It also streamlined the product development cycle, resulting in tasks being accomplished more swiftly and establishing a smooth workflow. It involved the development team more deeply in problem-solving throughout the process, providing timely, relevant feedback to avoid potential issues.
By embracing change as an integral part of software development, this approach fostered an innovative working environment. The result was a successful market launch of the Chain of Custody application within four months, marking a significant achievement in improving the pharmaceutical chain of custody.

Case Study

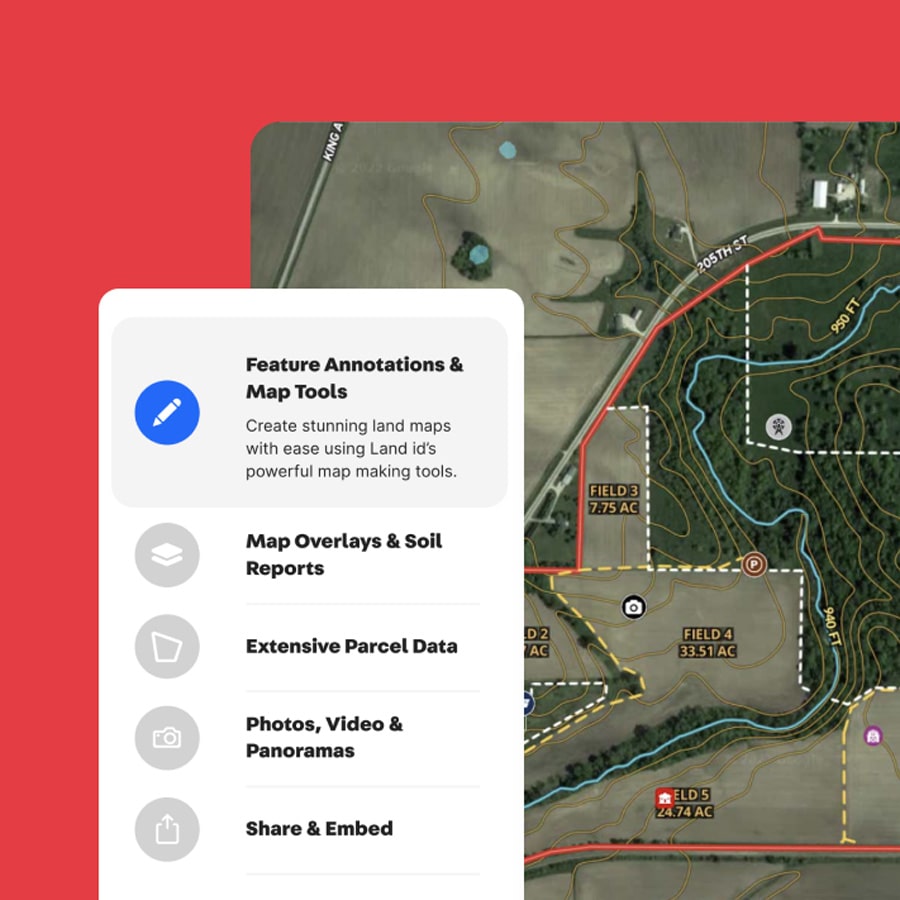
We partnered with Land id on their digital transformation, including completely redesigning their mobile application.


Case Study

We created an integrated breast health monitoring system, featuring a mapping device, an app with advanced dynamic AI algorithms and blockchain.



Case Study

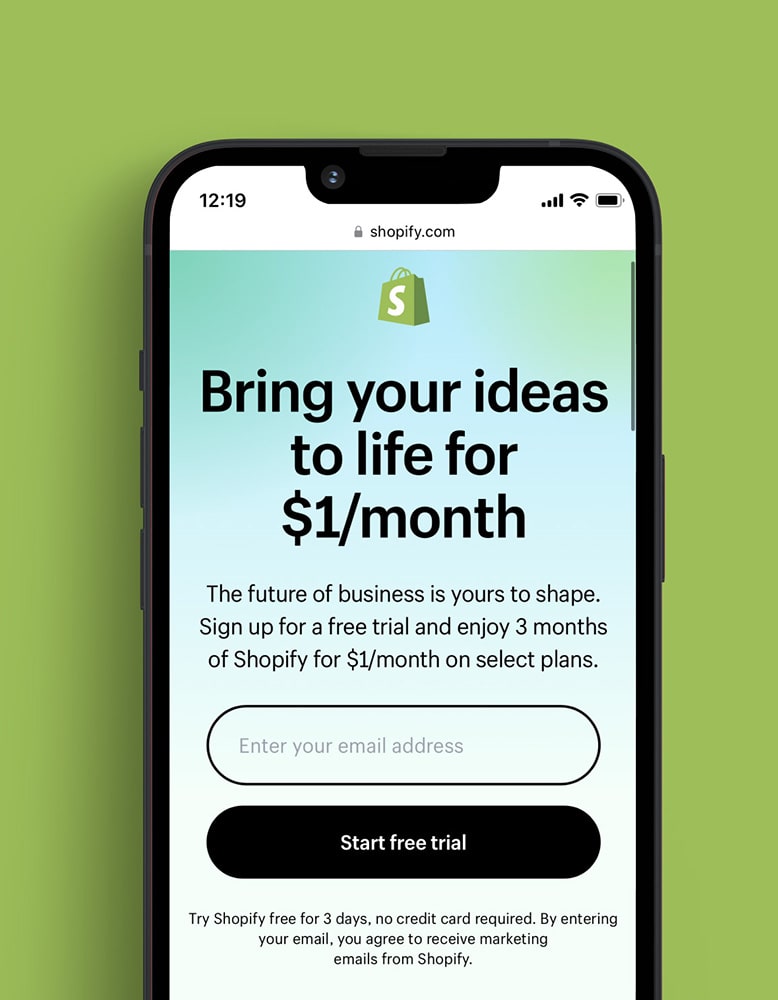
We worked together to create Shopify Ping, a mobile application for merchants to share links, offers, products descriptions, and more.


Case Study

Qubika provided UX/UI, mobile, desktop and web development services to take Brain.fm's platform to the next level.



Case Study

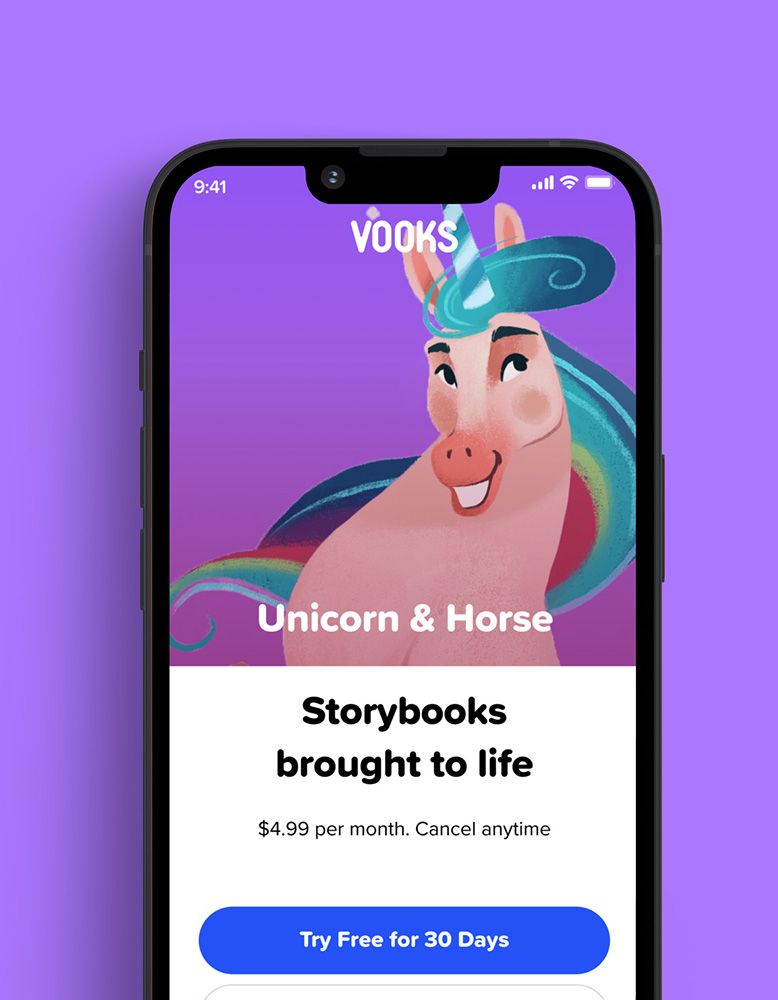
We worked side-by-side with Vooks to design and build a highly engaging, scalable streaming platform. The AWS environment was capable of accommodating hundreds of thousands of concurrent users.


Case Study
We highlight a range of case studies showing how we're using AI and computer vision technology to build innovative products that are transforming lives.



Case Study
Explore a range of case studies highlighting how Qubika is pushing forward the wearable revolution, and providing specific development services to our clients.



Case Study

OneSignal's strategic alliance with Qubika led to product diversification, platform integration, and rapid growth.



Case Study

Ripple partnered with Qubika to integrate crypto exchanges, expanding their services and enhancing global reach.



Get in touch with our experts to review your idea or product, and discuss options for the best approach
Get in touchArtificial Intelligence Services
Accelerate AI
Healthcare Solutions
Data
Agentic Factory
Financial Services Technology
Platform engineering
Data Foundation
AI Blog Post